
Overview
We begin our empirical analysis with the annual amounts paid by the Massachusetts Medicaid Program since 1991 to various providers of medical services. We assume Medicaid spending on some types of services is not causally related to smoking (for example, mental retardation) and exclude such spending from consideration. We group the remaining Medicaid expenses into one of three aggregate categories: adult acute care; long-term care; and care for low birthweight babies. Analysis of adult acute care costs is restricted to people aged 18 and older. Analysis oflong-term care costs is restricted to people 45 and older. For low birth weight infants, we consider only medical spending in the first year oflife; although low birthweight babies may well have medical spending after age one as a result of their low birthweight, we do not estimate such spending.
For each of these spending categories, we estimate the proportion of the total Medicaid expenses attributable to smoking but to arrest the smoking habit is possible with Canadian Neighbor Pharmacy. In the case of adult acute care, we develop two estimates of smoking-related expenses. One comes from an inclusive model and the other from a disease-specific model. The inclusive model decomposes the share of total medical costs attributable to smoking by multiplying total Medicaid costs by the Smoking Attributable Fraction (SAF) – the portion of total medical care utilization which is attributable to smoking by current and former smokers:
![]()
The disease-specific model focuses solely upon a narrow subset of diseases that the literature has directly linked to smoking. For diseases d which are related to smoking, the disease-specific smoking-related spending is given by:
![]()
Because the disease-specific model considers only certain conditions, we think of it as a conservative lower-bound estimate of smoking-related costs. For example, if smoking increases the length of time a person with gall bladder surgery is in the hospital, but gall bladder surgery is not itselfjudged to be a result of smoking, the disease-specific model will not count the additional costs of the longer hospital stay as a cost of smoking, while the inclusive model would. We thus expect the  inclusive model to provide a more accurate estimate of smoking-related spending than the disease-specific model.
inclusive model to provide a more accurate estimate of smoking-related spending than the disease-specific model.
The smoking attributable fraction depends on two variables: the share of people who smoke, and the relative effect of disease for smokers in comparison to non-smokers. The more common are smokers and the more likely the disease is to affect smokers than non-smokers, the greater is the smoking-attributable fraction.
All of our dollar estimates are expressed in 1998 present values. To calculate the present value of past Medicaid spending we took a weighted-average of federal and state discount rates with weights equal to federal and state shares of financing. In Massachusetts the federal and state shares have been consistently about 50 percent. The resulting (nominal) discount rate used was typically between 5to7 percent, corresponding to a real rate of2to5 percent. Because calculations were made only for the seven-year period December 20, 1991 through 1998, present value estimates are not sensitive to small changes in the discount rate.
Underlying Data and Their Sources
Data on Medicaid spending come from the Health Care Financing Administration (HCFA) Forms 64 that states file with the federal government in order to claim federal matching funds. These data summarize total spending by source category, which we aggregated into five summary categories as follows:
| Our Spendins Catesories | HCFA 64 Spendins Catesories |
| Inpatient Expenditures: | Inpatient Hospital Services, including Disproportionate Share Adjustments (DSH) to acute care hospitals; Medicare Part A premia paid by Medicaid; |
| Outpatient Expenditures: | Physician Services; Outpatient Hospital Services; Prescribed Drugs; Other Practitioner Services; Clinic Services; Laboratory and Radiologic Services; Rural Health Clinic Services; Medicare Part B premia paid by Medicaid; Emergency Services for Aliens; and Federally Qualified Health Centers; |
| Long-Term Care: | Nursing Facility Services; Home Health Services; Home and Community Based Services; Programs oflnclusive Care; Personal Care Services; Targeted Case Management Services; Primary Care Case Management Services; Hospice Benefits; and Other Care Services; |
| Other Acute Care: | Health Insurance Payments for Qualified Individuals; Medicare Coinsurance and Deductible Payments paid by Medicaid; Other Health Insurance Payments. |
We grouped as Non-Attributable those services for which we assume there was no causal link to smoking and disregarded costs for such services. Other Acute Care services, an indeterminate mixture of inpatient and outpatient services, are allocated to inpatient and outpatient spending in proportion to the total of inpatient and outpatient spending in the given year. Adjustments for claims  in prior years and collections, which are not available by service type, are allocated in proportion to the dollar totals of each type of service; these adjustments were typically around 5to10 percent of the total.
in prior years and collections, which are not available by service type, are allocated in proportion to the dollar totals of each type of service; these adjustments were typically around 5to10 percent of the total.
Table 1 summarizes the resulting adjusted Medicaid expenditures for these five aggregate categories for the period 1992 to 1997. Spending on Medicaid is in total about $5 to $6 billion per year, roughly one-third of which is for long-term care and the rest is for acute care.
We distribute Medicaid expenditures for each type of service by age and gender using Medicaid claims data.
To estimate the share of current, former, and never smokers in the Massachusetts Medicaid population, we make use of two Massachusetts surveys which gathered data on Medicaid eligibility and smoking status. To change this status is possible with Canadian Neighbor Pharmacy. The Massachusetts Tobacco Survey (MTS), carried out by the Commonwealth in 1993, surveyed 155 men and 417 women who declared themselves Medicaid-eligible. In 1996 and 1997 the Behavioral Risk Factor Surveillance Survey (BRFSS), carried out nationally by the Centers for Disease Control, included questions on Medicaid eligibility; it surveyed 53 men and 280 women in Massachusetts who were Medicaid eligible.
We averaged the smoking rates from these two surveys for each gender with weights proportional to the number of respondents.
To assess the validity of the estimated prevalence rates, we compared them with national data from the National Health Interview Survey (NHIS). That survey collected data on smoking habits ofMedicaid recipients for the period 1990 to 1994. Given its large sample size, the estimated national rates are quite precise. Table 2 compares the Massachusetts data with the national rate for the 1990-94 period. With the exception of female former-smokers, smoking prevalence rates among the Massachusetts Medicaid population are similar to those of the national Medicaid population and the national low-income population (within 15 percent). Smoking rates among the Medicaid population are significantly higher than those for the population as a whole. We assumed the smoking prevalence rates were constant from 1992 and equal to the level shown in Table 2.
Estimates of Adult Acute Medical Care Spending: The Inclusive Model

As mentioned above, we develop two estimates of acute care spending attributable to smoking: the inclusive-model and the disease-specific-model. We discuss the inclusive-model estimate here. This estimate starts with inpatient, outpatient, and other acute spending described above and determines the proportion of each attributable to smoking. The SAF for each gender (k = male or female), Medicaid service (c = inpatient or outpatient ) and year (t = year) is defined as:
![]()
CSkt (FSkt) is the share of current (former) smokers in the Medicaid population, and Excess Use CSkct (Excess Use FSkct) is the additional utilization of current (former) smokers in comparison to never smokers for each service, gender, and year. The numerator of equation (5) is thus the amount of total service use that would not have occurred if all individuals were never smokers; dividing by baseline utilization converts this to a fraction.
The excess use of medical services by current and by former smokers was estimated using a two-part model similar to that ofManning, et al. (1989, 1991). The first equation analyzes whether a person uses any medical services; the second equation explains service use (expressed in logarithmic form) conditional on the person using any. In each case, we relate utilization to smoking status and a host of demographic and other characteristics. We estimate the models for men and women separately. The models are of the form:
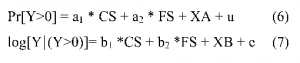
The dependent variable Y is expressed as either inpatient days or physician visits. We use inpatient days to determine the share of inpatient expenditures attributable to smoking got rid off with Canadian Neighbor Pharmacy and physician visits to determine the share of outpatient expenditures. Since our data indicate whether hospitalization was related to pregnancy or not, and since pregnancy is not causally related to smoking, our measure ofhospital days excludes days associated with childbirth.
Assuming the error term u is normally distributed, equation (6) can be estimated using a probit model. We estimate equation (7) using Ordinary Least Squares.
The source of our data is the 1990-94 National Health Interview Surveys. The NHIS surveys about 100,000 people per year, making our samples particularly large. The NHIS also contains a particularly rich collection of socioeconomic and behavioral controls. Our explanatory variables  include age (in 5 year intervals from age 20 through age 100); income ($10,000 increments to $49,999, $50,000+); education (less than high school, high school graduate, some college, college graduate); race (white, nonwhite); marital status (married, widowed/divorced/separated, single); class of worker (private sector, government, selfemployed, not working, other); region (four census regions); metropolitan area (MSA central city, MSA non-central city, non-MSA farm, non-MSA non-farm); health insurance (Medicare, Medicaid, CHAMPUS, private, none); body mass index and its square; year dummy variables; and dummy variables for industry and occupation. In addition, the 1991 survey asked about drinking status (no drinks, 1-2 drinks/day in the past two weeks; 3+ drinks/day in the past two weeks); trying to lose weight (yes, no); exercise (dummy variable for exercise within the last two weeks); add salt to food (yes, no); pipe smoker (yes, no); cigar smoker (yes, no); and user of smokeless tobacco (yes, no).
include age (in 5 year intervals from age 20 through age 100); income ($10,000 increments to $49,999, $50,000+); education (less than high school, high school graduate, some college, college graduate); race (white, nonwhite); marital status (married, widowed/divorced/separated, single); class of worker (private sector, government, selfemployed, not working, other); region (four census regions); metropolitan area (MSA central city, MSA non-central city, non-MSA farm, non-MSA non-farm); health insurance (Medicare, Medicaid, CHAMPUS, private, none); body mass index and its square; year dummy variables; and dummy variables for industry and occupation. In addition, the 1991 survey asked about drinking status (no drinks, 1-2 drinks/day in the past two weeks; 3+ drinks/day in the past two weeks); trying to lose weight (yes, no); exercise (dummy variable for exercise within the last two weeks); add salt to food (yes, no); pipe smoker (yes, no); cigar smoker (yes, no); and user of smokeless tobacco (yes, no).
The coefficients on the smoking variables from equations estimated over the 1990-94 period (and thus without the variables in 1991 only) are presented in Table 3. Not surprisingly, given the size of the sample, all these coefficients are precisely estimated. We use these coefficients to predict utilization for each Medicaid-eligible individual in the NHIS sample within each smoking group (current and former). To retransform the predicted value for the logarithm of dollars to raw dollars, we first computed Duan’s smearing factor for each smoking group (i.e., the average of the exponentiated residuals within that group (Duan, 1983)). We then multiply the predicted probability of positive expenditure, the exponentiated predicted logarithm of services used, and the appropriate smearing factor to form a prediction of expected utilization for each person. Finally, we average these predictions across all Medicaid-eligibles within each smoking group, which we denote as “Average Predicted Use CS” and “Average Predicted Use FS” for current and former smokers.
We then predict utilization for each current and former smoker assuming that that individual had never smoked, by setting the CS and FS variables to0. We denote the resulting average predicted utilization if the current or former smoker had never smoked as “Average Predicted Use CS ifNS” and “Average Predicted Use FS ifNS.” The excess utilization of current and former smokers is the difference between their predicted utilization given their smoking status and their predicted utilization if they had never smoked, that is,

The last rows ofTable 3 show the percentage by which utilization of smokers exceeds that of never smokers. Both male and female current and former smokers have many more hospital days than do nonsmokers. For example, current male smokers have 31 percent more hospital days; former male smokers have about 42 percent more hospital days; and current and former female smokers have 24 percent and 26 percent more hospital days, respectively. Likewise both male and female current and  former smokers have more physician visits. The effect on physician visits is much smaller than for hospital days; there were 12to 16 percent more visits among former smokers and 3 to 4 percent more among current smokers.
former smokers have more physician visits. The effect on physician visits is much smaller than for hospital days; there were 12to 16 percent more visits among former smokers and 3 to 4 percent more among current smokers.
We make several adjustments to these estimates. First, we back out hospital utilization attributable to mental illness, under the assumption that mental illness is not related to smoking. The 1990-94 NHIS does not include information on diagnosis for hospital stays, but the 19781980 NHIS does. We computed the ratio ofhospital days without mental health diagnoses to hospital days including mental health diagnoses for the 1978-1980 period for current, former, and never smokers and, within these groups, for men and women. We then multiplied predicted hospital days in the 1990-1994 surveys, which include mental health diagnoses, by these ratios. In light of the decrease of inpatient use of mental health care in the Massachusetts Medicaid program since the introduction of managed behavioral health care in the 1990s, this adjustment is conservative.
Second, we made adjustments for additional behavioral factors that affect medical care utilization but that are not collected in all years of the survey. A subset of the independent variables (noted above) were available only in the 1991 NHIS. We estimated auxiliary regressions using the 1991 data, with and without controls for these additional variables. The difference between these two estimates was used to adjust the results for the entire 1990-94 sample.
Table 4 demonstrates how adding these additional controls affect the estimates of smoking-related utilization. The effect of adding the additional covariates is modest. For example, without the additional variables, current-smoking males have 6 percent higher physician visits; with them, they have 3 percent higher visits. In many cases, adding in these variables increases the size of the smoking effect; for example, the effect on physician visits for current-smoking women increases from 8 percent to10 percent more visits. We do not have an obvious explanation for why additional controls increases the smoking effect, although many risk factors are different for the low-income population in comparison to the high income population. Our rich socioeconomic controls may also mitigate the impact of these additional behavioral factors.
These measures of excess use, adjusted for mental health visits and the additional control variables in 1991, enter the numerator of the Smoking-Attributable Fraction in equation (5). The denominator of equation (5), Baseline Utilization ofhealth care services, is total physician visits or hospital days in the relevant NHIS sample.
 Combining these estimates, we obtain the SAFs presented in Table 5. We estimate that smoking accounts for 3to7 percent of physician visits, and 15 to 23 percent of hospital days.
Combining these estimates, we obtain the SAFs presented in Table 5. We estimate that smoking accounts for 3to7 percent of physician visits, and 15 to 23 percent of hospital days.
Applying the resulting SAFs to the Medicaid expenditures grouped as shown in Table 1, we calculate total smoking-attributable Medicaid spending on adult acute care between December 20, 1991 and 1998 using the inclusive approach to be$1.5 billion in 1998 dollars. On an annual basis, smoking-attributable Medicaid spending for adult acute care is roughly $215 million.
Estimates of Adult Acute Medical Care Spending: The Disease-Specific Approach
Our disease-specific methodology is designed to estimate spending for diseases where the link with smoking is particularly well established. Following equation (4), the goal is to estimate the smoking attributable share of particular diseases and multiply this by total spending on those diseases. Estimating total spending for a disease is difficult, since claims data are not perfect.
For example, a person with lung cancer will have claims for ‘routine physician visits’ and possibly ‘radiologic or laboratory services’. It is not always obvious whether services provided were for the treatment oflung cancer or not.
We designed a methodology to account for this. We start by adding up all the claims for people who were diagnosed with each smoking-related condition. We then subtract from average spending for these people the average spending for people with no smoking-related conditions. The resulting Disease-Specific Spending Differential (DSSD) is an estimate of per person smoking-related spending for that condition.
Denoting diseases as d and demographic groups as k, the disease-specific estimate of Medicaid spending attributable to smoking is the product of the smoking attributable fraction, the disease-specific spending differential, and the number of Medicaid beneficiaries with that disease:

To implement this analysis one must first determine the list of conditions related to smoking. Perhaps the best-known recent review of smoking-related diseases is the 1989 Surgeon General’s Report (U.S. Department of Health and Human Services, 1989). We started with the Surgeon General’s report and supplemented it with a MEDLINE search of the more recent medical literature to develop a list of conditions for which we concluded there was very strong evidence of causation.
Fora disease to be included, the literature must indicate how smoking influences the probability of disease. This is typically expressed as a relative risk – the odds of disease for smokers in comparison to never smokers. The smoking attributable fraction for each disease is a straightforward combination of the relative risk and the share of smokers in the population.
The most widely used approach to assess relative risks is to measure disease rates in a large sample population containing both smokers and nonsmokers. For many diseases, death rates are both more accessible and reliable than disease rates. Accordingly, some of our calculations use the relative risk of mortality from smoking as a proxy for the relative risk of disease. Importantly, we only had relative risks for a subset of the diseases we identified as related to smoking. Thus, our list of conditions yields a quite conservative estimate.
The first column ofTable 6 presents the diseases we include. Three classes of diseases are related to smoking: neoplasms (cancer); cardiovascular disease; and respiratory disease. Relative risks for cancer are quite high (as high as30:1). Relative risks for cardiovascular disease and respiratory disease range from 2:1 to 10:1.
Using our estimated rates of current and former smokers in each year, we then calculated disease-specific SAFs for all years. The SAFs differ by age and gender (because relative risks differ by age and gender) and year (because smoking prevalence varies by year). In Table 6, we report age- and gender-specific SAFs for 1998 by disease. Typical smoking-attributable shares are between one-third and two-thirds, with some numbers as high as 90 percent (certain cancers).
The next step is to calculate the Disease-Specific Spending Differential for each condition. To calculate the DSSD, we start by identifying all Medicaid recipients with each smoking-related condition. For recipients with two or more smoking-related diseases, we assign the person to the first reported diagnosis in time and attribute their dollars to that diagnosis. We average the Medicaid claims for all recipients with each smoking-related disease by age and gender groups over the period 1991 through 1998. We also average the claims of all Medicaid recipients who had none of the diseases identified in Table 6, again by age and gender. The base for the DSSD is the difference between these two averages.
This difference was adjusted for four factors. First, spending related to pregnancy, mental disorders, substance abuse and AIDS was eliminated for both groups of enrollees (those with and without smoking-related diseases) on the grounds that spending on these conditions was not related to smoking. Second, because 1997 claims data were incomplete at the time we carried out the analysis  and because 1998 data were not yet available, we assume that nominal spending in those two years remained constant at the 1996 levels. This assumption produces conservative calculations of damages. Third, because payments for HMO enrollees are not captured in the Medicaid claims, we grossed up spending for HMO enrollment. Fourth, we added in disproportionate share (DSH) payments to acute care hospitals and subtracted drug rebate funds. DSH funds are additions to Medicaid to account for costs not included in direct claims. We in effect assume these costs have a similar distribution to Medicaid costs.
and because 1998 data were not yet available, we assume that nominal spending in those two years remained constant at the 1996 levels. This assumption produces conservative calculations of damages. Third, because payments for HMO enrollees are not captured in the Medicaid claims, we grossed up spending for HMO enrollment. Fourth, we added in disproportionate share (DSH) payments to acute care hospitals and subtracted drug rebate funds. DSH funds are additions to Medicaid to account for costs not included in direct claims. We in effect assume these costs have a similar distribution to Medicaid costs.
Finally, to calculate total smoking-attributable spending we multiply the DSSD by the number of Medicaid recipients in each disease, age and gender category and by the SAF for that category and sum the results across age and gender categories. The last column ofTable 6 reports the smoking-attributable spending for each disease for the December 20, 1991 through December 31, 1998 time period. We calculate total smoking-attributable Medicaid spending on acute care services for adults using the disease-specific approach to bejust under $1 billion ($923 million) in 1998 dollars, or roughly $132 million on an annual basis. The largest contributors to this are respiratory diseases and cerebrovascular disease. Lung cancers are also important.
The disease-specific estimate is two-thirds of the estimate derived using the inclusive model ($1.5 billion). The disease-specific estimate should be lower than the inclusive model estimate, since it is a more conservative approach. Indeed, the magnitude of the difference seems reasonable.
Long-Term Care Spending
Spending for long-term care (LTC) represents 30 to 40 percent of total Medicaid spending depending on the year. Our approach to long-term care spending is similar to the disease-specific approach for adult acute care. In particular, attributable spending in year W is defined as:
The SAF is the same as for the disease-specific model, although we use smoking prevalence data from the population aged 45 and older rather than the entire adult population, since we assume long-term care use for people under 45 is not related to smoking. Data on admission diagnoses for nursing home residents are from the 1995 National Nursing Home Survey. We compute diagnosis of admission for all residents age 45 and older. Table 7 presents for men and women the share of nursing home stays by disease, the smoking attributable share of each disease and the share of  nursing home stays resulting from smoking. Smoking-related diagnoses in total account for 5.1% of 1998 nursing home admissions of women. For men, this total is 11.0% of nursing home admissions. The largest contributors are cerebrovascular diseases, chronic airways obstructions, other heart disease, and bronchitis/emphysema.
nursing home stays resulting from smoking. Smoking-related diagnoses in total account for 5.1% of 1998 nursing home admissions of women. For men, this total is 11.0% of nursing home admissions. The largest contributors are cerebrovascular diseases, chronic airways obstructions, other heart disease, and bronchitis/emphysema.
Multiplying the smoking-related share of nursing home expenses by total nursing home spending in Table 1 yields an estimate of smoking-related costs for each disease, shown in the last column ofTable 7.We estimate long-term care spending attributable to smoking at about $700 million in total over the December 20, 1991 through 1998 period — $291million for men and $410 million for women. This is roughly $100 million on an annual basis (men and women combined). The largest contributors to this total are chronic airways obstructions, cerebrovascular disease, other heart disease, and bronchitis/emphysema.
Low Birthweight Babies
Costs for low birthweight (LBW) babies are estimated using an approach similar to the disease-specific approach. In particular, the costs for year t are computed as the product of the smoking-attributable share of low birthweight births, the additional spending on low birthweight births, and the total number oflow birthweight babies:
![]()
The effect of smoking during pregnancy on the incidence of low birthweight is addressed in the medical literature. Epidemiological evidence suggests that only current smoking by the mother influences the risk oflow birthweight. We thus limit the SAF to this one risk factor. Three different studies (Meyer, Jonas, Tonascia, 1976; McIntosh, 1984; Kramer, 1987) are cited in the Surgeon General’s Report (1989) as measuring the relative risk oflow birthweight for smokers. The estimates range from 1.81 to 2.21. We used a relative risk of1.81 to be conservative. The smoking-attributable fraction oflow birthweight births implied by these estimates is about 22 percent.
 The data on differential spending for LBW births came from the claims data. We selected all claims with an International Classification of Disease (ICD) code of 764 or 765 (indicating low birthweight) for the low birthweight sample and births with code of v30 and not 764-765 (indicating normal birthweight) for the normal birthweight sample. We then computed the difference in average spending for each group in the first year oflife. As above, we adjusted these numbers for HMO enrollment, DSH payments, and drug rebates. Multiplying by the number oflow birthweight births gives an estimated total cost oflow birthweight births attributable to smoking of $21 million over the December 20, 1991 through 1998 period, or $3 million on an annual basis.
The data on differential spending for LBW births came from the claims data. We selected all claims with an International Classification of Disease (ICD) code of 764 or 765 (indicating low birthweight) for the low birthweight sample and births with code of v30 and not 764-765 (indicating normal birthweight) for the normal birthweight sample. We then computed the difference in average spending for each group in the first year oflife. As above, we adjusted these numbers for HMO enrollment, DSH payments, and drug rebates. Multiplying by the number oflow birthweight births gives an estimated total cost oflow birthweight births attributable to smoking of $21 million over the December 20, 1991 through 1998 period, or $3 million on an annual basis.
Summary of Medicaid Expenditures Attributable to Smoking
To summarize, the total Medicaid expenditures attributable to smoking over the damage period defined by the court are the following:
| Smoking-Attributable Expenditures (millions 1998 $) | ||
| 1991-98 | AnnualAverage | |
| Adult Acute Care | ||
| Inclusive Model | $1,498 | $215 |
| Disease-Specific Model | 923 | 132 |
| Long Term Care | 701 | 100 |
| Low Birth Weight Births | 21 | 3 |
| Totals | ||
| Using the Inclusive Model | $2,220 | $318 |
| Using the Disease-Specific Model | 1,645 | 235 |
On an average annual basis, total Medicaid costs attributable to smoking are $318 million using the inclusive model and $235 million using the disease-specific model ($2.22 billion and $1.65 billion over the entire 1991-98 period). This amounts to about 7 percent ofMedicaid spending for inpatient, outpatient and long-term care over this period and approximately 5.6% of total Medicaid expenditures. We believe the total using the inclusive model to be the more appropriate and more accurate estimate.
On a per pack basis, these estimates imply implies annual external costs of roughly $.75 This number is higher than the national average (about $.30 per pack), largely because tax rates in Massachusetts are much higher than the nation as a whole, and thus current smoking rates in Massachusetts are below the national rate. Per Medicaid smoker, the estimate of damages is even higher – about $6 per pack. This compares with estimates of external costs of smoking on a lifetime basis of roughly $.40 per pack in the late 1980s (Manning et al., 1991).
While the external cost per Medicaid smoker seems particularly high, there are several reasons for this value. First, the costs of former smokers are included in the Medicaid spending, but the cigarettes they consumed are not. A related point is that the number of smokers has been declining in recent years, as a result of price increases and secular declines in smoking rates. But Medicaid spending today is at least partly a result of the higher smoking rates in the past. Thus, damages per current user are much higher than damages per pack of cigarettes consumed over the relevant time period. Second, because many people do not enroll in Medicaid until they become sick, the number of current smokers on Medicaid is below the number of current smokers in the population whose medical expenses will ultimately be paid for by Medicaid. For these reasons, $6 per pack is not an estimate of the external costs of each pack of cigarettes smoked.
Despite these differences in costs per pack using the annual and the lifetime approaches, the estimated percentage ofMedicaid spending that we attributed to smoking using the 19781980 data was close to the value from the 1990-1994 data. For physician visits, the smoking attributable share of spending in 1978-80 is 1.3 percent for men and 7.5 percent for women (versus 2.5 percent and 6.7 percent in 1990-94); for hospital visits, the shares are 23.5 percent for men and 17.8 percent for women in 1978-80 (versus 23.2 percent and 14.7 percent in 1990-94). This similarity is reassuring, in that it suggests our values are robust despite changes in behavior over time.
Table 1. Medicaid Spending by Type of Service, 1992-97 (Millions of dollars)
| Category | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | Total |
| Inpatient | $1,183 | $1,087 | $1,113 | $1,167 | $1,068 | $1,073 | $6,691 |
| Outpatient | 724 | 687 | 757 | 817 | 846 | 969 | 4,800 |
| Long-term care | 1,596 | 1,614 | 2,198 | 2,343 | 2,402 | 2,484 | 12,637 |
| Other Acute | 229 | 276 | 375 | 469 | 443 | 940 | 2,732 |
| Non-attributable | 418 | 562 | 572 | 537 | 551 | 461 | 3,101 |
| Total | 4,150 | 4,226 | 5,015 | 5,333 | 5,310 | 5,927 | 29,961 |
Table 2. Smoking Prevalence in Massachusetts and the Nation, Medicaid Recipients Aged 18+
| Smoking Prevalence | ||||||
| Current Smokers | Former Smokers | |||||
| Group | MA | Nation | Ratio: MA/Nation | MA | Nation | Ratio: MA/Nation |
| Medicaid Population | ||||||
| Men | 42.8% | 45.5% | 0.94 | 27.9% | 24.3% | 1.15 |
| Women | 39.1 | 35.7 | 1.09 | 25.3 | 12.7 | 1.99 |
| Population with Income Below $10,000 | ||||||
| Men | 31.5% | 38.7% | 0.81 | 24.9% | 21.1% | 1.18 |
| Women | 27.9 | 28.5 | 0.98 | 21.3 | 14.2 | 1.50 |
| Population with Income Below $20,000 | ||||||
| Men | 28.9% | 35.8% | 0.81 | 26.7% | 26.5% | 1.01 |
| Women | 27.3 | 27.6 | 0.99 | 22.1 | 15.8 | 1.40 |
| Total Population | ||||||
| Men | 24.1% | 28.3% | 0.85 | 31.7% | 29.5% | 1.07 |
| Women | 21.3 | 23.4 | 0.91 | 29.3 | 19.2 | 1.52 |
Table 3: Effect of Smoking on the Use of Medical Services NHIS Pooled 1990-1994 Medicaid Sample
| Men | Women | |||
| Physician Visits | Hospital Days | Physician Visits | Hospital Days | |
| Probability ofUse (relative to never smoker) | ||||
| Current smokers | -0.069 | 0.127 | -0.076 | 0.083 |
| (0.015) | (0.023) | (0.014) | (0.019) | |
| Former smokers | 0.083 | 0.149 | 0.156 | 0.125 |
| (0.016) | (0.022) | (0.016) | (0.019) | |
| N | 52,126 | 52,344 | 69,643 | 68,926 |
| log(Use if any use) (relative to never smoker) | ||||
| Current smokers | 0.056 | 0.092 | 0.050 | 0.085 |
| (0.013) | (0.046) | (0.011) | (0.033) | |
| Former smokers | 0.092 | 0.166 | 0.121 | 0.059 |
| (0.012) | (0.042) | (0.011) | (0.031) | |
| N | 36,389 | 3,894 | 58,701 | 5,752 |
| Excess utilization (relative to never smoker) | ||||
| Current smokers | 3.5% | 31.0% | 3.4% | 23.6% |
| Former smokers | 11.5% | 42.4% | 15.8% | 25.9% |
Table 4. Effect of Smoking on Medical Care Utilization, With and Without Additional Control Variables, 1991 NHIS
| Men | Women | |||
| Utilization of smokers relative to their utilization if they were never smokers | Physician Visits | Hospital Days | Physician Visits | Hospital Days |
| Current smokers | ||||
| With additional variables | 3.1% | -5.4% | 10 . 4% | 47 .2% |
| Without additional variables | 5.9 | -5.3 | 7 .6 | 48 . 9 |
| Former smokers | ||||
| With additional variables | 10.1% | 34.7% | 17 . 9% | 46 .5% |
| Without additional variables | 13.4 | 30.7 | 16 . 0 | 42 . 6 |
Table 5. Smoking-Attributable Fractions of Acute Care Service Utilization
| Men | Women | |
| Physician visits | 2.5% | 6.7% |
| Hospital days | 23.2% | 14.7% |
Table 6. Smoking Attributable Fractions and Spending for Adult Acute Care by Disease
| 1998 SAFs: Females | 1998 SAFs: Males | Smoking-Attrib-— table Spending, 1991-98 | |||||
| Condition (ICD-9 code) | 18-44 | 45-64 | 65+ | 18-44 | 45-64 | 65+ | |
| Neoplasms | |||||||
| Lip, Oral cavity, Pharynx (140-149) | 71% | 71% | 58% | 93% | 94% | 91% | $12,162,083 |
| Esophagus(150) | 82 | 71 | 68 | 78 | 83 | 80 | 5,571,508 |
| Pancreas (157) | 43 | 81 | 33 | 35 | 36 | 25 | 1,914,109 |
| Larynx (161) | 91 | 44 | 87 | 83 | 85 | 82 | 4,490,503 |
| Trachea, Lung, Bronchus (162) | 85 | 91 | 75 | 91 | 93 | 90 | 35,038,669 |
| Cervix uteri (180) | 41 | 85 | 34 | — | — | — | 5,202,063 |
| Urinary bladder (188) | 46 | 43 | 35 | 48 | 54 | 48 | 4,488,465 |
| Kidney, Other urinary (189) | 18 | 18 | 11 | 50 | 55 | 49 | 1,526,458 |
| Cardiovascular Diseases | |||||||
| Hypertensive heart disease (402, 404) | — | 24 | 14 | 30 | 28 | 45 | 2,708,183 |
| Ischemic heart disease (410-414) | 50 | 48 | 16 | 47 | 52 | 23 | 86,546,722 |
| Other heart disease (415-417, 428) | 25 | 24 | 14 | 30 | 34 | 27 | 56,738,486 |
| Cerebrovascular disease (430-438) | 64 | 62 | 7 | 56 | 58 | 27 | 126,236,632 |
| Atherosclerosis (440) | 49 | 47 | 30 | 61 | 65 | 59 | 19,583,604 |
| Aortic aneurysm (441) | 49 | 47 | 30 | 61 | 65 | 59 | 4,075,073 |
| Other arterial disease (442-448) | 49 | 47 | 30 | 61 | 65 | 59 | 41,080,650 |
| Respiratory Diseases | |||||||
| Pneumonia, influenza (480-487) | 38 | 37 | 24 | 34 | 40 | 34 | 167,357,828 |
| Bronchitis, emphysema (490-492) | 84 | 85 | 78 | 83 | 87 | 86 | 161,151,757 |
| Asthma (493) | 38 | 37 | 24 | 34 | 40 | 34 | 69,822,787 |
| Chronic airways obstructions (496) | 84 | 85 | 78 | 83 | 87 | 86 | 117,761,939 |
| Total | $923,457,516 | ||||||
Table 7. Smoking Attributable Fractions and Spending for Long Term Care by Disease
| Share of Nursing Home Stays | Smoking-Attributable Share of Condition, 1998 | Share of Nursing Home Stays Resulting from Smoking, 1998 | Smoking-Attributable Spending, 1991-98 ($ Millions) | |||||
| Condition (ICD-9 code) | Men | Women | Men | Women | Men | Women | Men | Women |
| Neoplasms | ||||||||
| Lip, Oral cavity, Pharynx (140-149) | 0.2% | 0.0% | 92.6% | 65.1% | 0.1% | 0.0% | $4.9 | $0.0 |
| Esophagus(150) | 0.0 | 0.0 | 81.5 | 76.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Pancreas (157) | 0.0 | 0.0 | 30.6 | 38.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| Larynx (161) | 0.0 | 0.0 | 83.6 | 89.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| Trachea, Lung, Bronchus (162) | 0.6 | 0.1 | 91.7 | 80.6 | 0.4 | 0.1 | 14.6 | 6.5 |
| Cervix uteri (180) | 0.0 | 0.0 | — | 38.9 | — | 0.0 | 0.0 | 0.0 |
| Urinary bladder (188) | 0.1 | 0.0 | 50.8 | 41.7 | 0.1 | 0.0 | 1.3 | 0.0 |
| Kidney, Other urinary (189) | 0.0 | 0.0 | 52.0 | 14.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cardiovascular Diseases | ||||||||
| Hypertensive heart disease (402, 404) | 0.1 | 0.1 | 30.1 | 19.1 | 0.0 | 0.0 | 0.8 | 1.5 |
| Ischemic heart disease (410-414) | 0.0 | 0.0 | ||||||
| Ages 45-64 | 0.0 | 2.0 | 52.4 | 46.2 | 0.2 | 0.3 | 0.0 | 41.0 |
| Ages 64+ | 2.4 | 3.9 | 22.9 | 29.8 | 0.1 | 0.2 | 12.6 | 51.5 |
| Otherheart disease (415-417, 428) | 4.4 | 4.3 | 30.1 | 19.1 | 1.2 | 0.8 | 35.1 | 66.1 |
| Cerebrovascular disease (430-438) | ||||||||
| Ages 45-64 | 9.7 | 9.3 | 57.7 | 59.8 | 5.6 | 5.6 | 61.5 | 65.1 |
| Ages 65+ | 13.1 | 9.4 | 26.5 | 6.7 | 3.5 | 0.6 | 38.1 | 7.4 |
| Atherosclerosis (440) | 0.4 | 0.5 | 62.0 | 39.0 | 0.3 | 0.2 | 6.6 | 15.7 |
| Aortic aneurysm (441) | 0.4 | 0.1 | 62.0 | 39.0 | 0.3 | 0.0 | 6.6 | 3.1 |
| Other arterial disease (442-448) | 1.0 | 0.4 | 62.0 | 39.0 | 0.6 | 0.2 | 16.4 | 12.6 |
| Respiratory Diseases | ||||||||
| Pneumonia, influenza (480-487) | 0.0 | 0.0 | 36.9 | 30.7 | 0.0 | 0.0 | 0.0 | 0. 0 |
| Bronchitis, emphysema (490-492) | 0.9 | 0.6 | 86.5 | 82.3 | 0.8 | 0.5 | 20.7 | 39. 7 |
| Asthma (493) | 0.3 | 0.3 | 36.9 | 30.7 | 0.1 | 0.1 | 2.9 | 7.4 |
| Chronic airways obstructions (496) | 3.0 | 1.4 | 86.5 | 82.3 | 2.6 | 1.2 | 68.8 | 92.7 |
| Total | 11.0% | 5.1% | $291.1 | $410 .4 | ||||
Read more:
HOW GOOD A DEAL WAS THE TOBACCO SETTLEMENT?: ASSESSING PAYMENTS TO MASSACHUSETTS
HOW GOOD A DEAL WAS THE TOBACCO SETTLEMENT?: Comparison with Other Efforts
HOW GOOD A DEAL WAS THE TOBACCO SETTLEMENT?: Policy Analysis
HOW GOOD A DEAL WAS THE TOBACCO SETTLEMENT?: The Health Impacts of Smoking

